The State of Artificial Intelligence in Medical Devices in 2021
The scope of software in healthcare has expanded exponentially in the last decade. The healthcare industry has been embracing exciting technological innovations more readily and one of the most recent examples of this is Artificial Intelligence being integrated (AI) into medical devices.
The future of healthcare lies in AI playing a significant role in developing precision medicine for correct diagnosis and personalized treatments plans. Medical companies are applying AI technology in medical devices for imaging, management of chronic diseases, and devices for patient care. In this article, we’re taking a closer look at the applications of AI in medical devices and how medical device companies are innovating their products to address the need for more accurate diagnosis.
Artificial Intelligence in the Medical Device Industry
As medical diagnoses rely on larger and larger data sets, healthcare systems are becoming more likely to be overwhelmed. With the help of medical devices with AI integration, there is finally a way to eliminate educated guesses and allow doctors to make more data-driven medical decisions to improve patient care.
This level of accuracy has led to some promising applications of AI in the industry that make the process of patient assessment faster, from consultation to prognosis.

1. Advanced Processing in Medical Imaging
The application of AI in diagnostic imaging and radiation therapy is seen to have numerous advantages. It could improve the quality of data images obtained through X-rays, MRI scanning, ultrasound, computed tomography, and nuclear medicine. Additionally, it can decrease radiation dosage in radiation therapy and segment pathology areas accurately.
2. Medical Device Software
The International Medical Device Regulators Forum officially defined the term “Software as a Medical Device” in a 2013 report, as “software intended to be used for one or more medical purposes that perform these purposes without being part of a hardware medical device.”
Since then, medical device software has expanded to include a wide range of processing software used in medical tools to provide diagnosis, therapy, or information to help medical practitioners make a final and accurate diagnostic decision.
Medical device software is subject to FDA regulations under the new Software as a Medical Device (SaMD) which acts as an action plan and offers guidelines to stakeholders. Medical software developers must also be aware of these guidelines to align the development of their products to meet quality standards.
3. Quality and Management Systems
Before a medical device enters the market, It needs to undergo several stages of quality management requirements. Since medical device manufacturing and distribution in the United States is overseen by FDA’s Center for Devices and Radiological Health (CDRH), devices must comply with seven basic regulatory requirements outlined by the CDRH:
- Establishment registration,
- Medical Device Listing,
- Premarket Notification 510(k), unless exempt, or Premarket Approval (PMA),
- Investigational Device Exemption (IDE) for clinical studies
- Quality System (QS) regulation,
- Labeling requirements, and
- Medical Device Reporting (MDR)
Additionally, to ensure compliance in international markets, organizations can use ISO 13485, which specifies the requirements of an effective quality management system for the production, design, and installation of medical devices.
4. After-Sales Monitoring
Creating a safe and quality product doesn’t stop at CDRH, ISO, and QMS compliance requirements. Medical devices require post-market surveillance to monitor product performance in an actual medical environment. The surveillance aims to verify the benefits, identify unknown risks, and analyze possible improvements for the subsequent product versions.
Regulations on Artificial Intelligence in Medical Devices
Before medical devices with AI applications can be made available in the US, they must undergo stringent testing and comply with the FDA regulations.
Although a lot of information around medical device AI isn’t publicly available at the moment, FDA has made its stance clear on the future of medical devices and AI’s part in it. The Center for Devices and Radiological Health (CDRH), a part of the FDA, recently announced the Digital Health Center of Excellence with numerous resources including a list of AI/ML-enabled medical devices marketed in the United States.
But the truth is that FDA has traditionally been more restrictive of new technologies than international regulatory bodies, particularly the EU MDR. EU MDR is the European equivalent of the FDA and shares numerous similarities in its goals, functions, and medical device regulations.
Related: EU MDR for US-based Medical Device Manufacturers and Distributors
The new guidelines address the limits and reporting requirements for at least 2,000 materials used in the design and manufacture of medical devices. Among the substances, machines, or parts of instruments covered by the EU MDR include the integration of artificial intelligence in the development and usage of medical devices and implants.
One of the main requirements of EU MDR for approving AI in medical devices is to ensure safety through risk management. Applying risk management in medical devices needs the expert assessment of a qualified medical physicist. They can assess the safety and performance of medical devices and provide accurate medical input to help make the device more compliant with EU MDR standards.
The updated EU MDR is also less focused on pre-approval regulations and has increased medical manufacturers’ responsibility throughout the product life cycle, making compliance requirements closer to that outlined by the FDA – making EU MDR more relevant to US-based medical companies than ever.

What Medical Manufacturers Need To Know
In a recently published study, researchers studied the implications of using artificial intelligence in the medical device industry as well as the role of medical physicists in it.
Manufacturers need to work hand-in-hand with Medical Physicists to assess the viability of the devices and ensure safety and accurate performance. Qualified medical physicists play a significant role in developing AI medical devices because their medical knowledge is the basis of the safety standards in the software. The MPs will assess the clinical decision-making output of the devices to ensure quality and accuracy. They should be an integral part of the design of the devices to ensure precision from the beginning.
The MPs, therefore, should also be well-informed of the most current regulations of the FDA CDRH and EU MDR in medical devices. Knowing will give them direction, aligned with the level of restrictions required by the regulators. They can help develop medical devices that can be approved for release in both domestic and external markets.
How SurgiCloud Helps
Medical manufacturers are gearing their innovations towards deep learning and artificial intelligence because of their powerful use cases in the medical industry. Not to mention the stringent regulations each medical device will have to undergo to get a CE marking to prepare it for commercialization. However, the future of medicine is towards automation and precision, and where all efforts should be.
Begin your journey towards automation and precision with Surgicloud
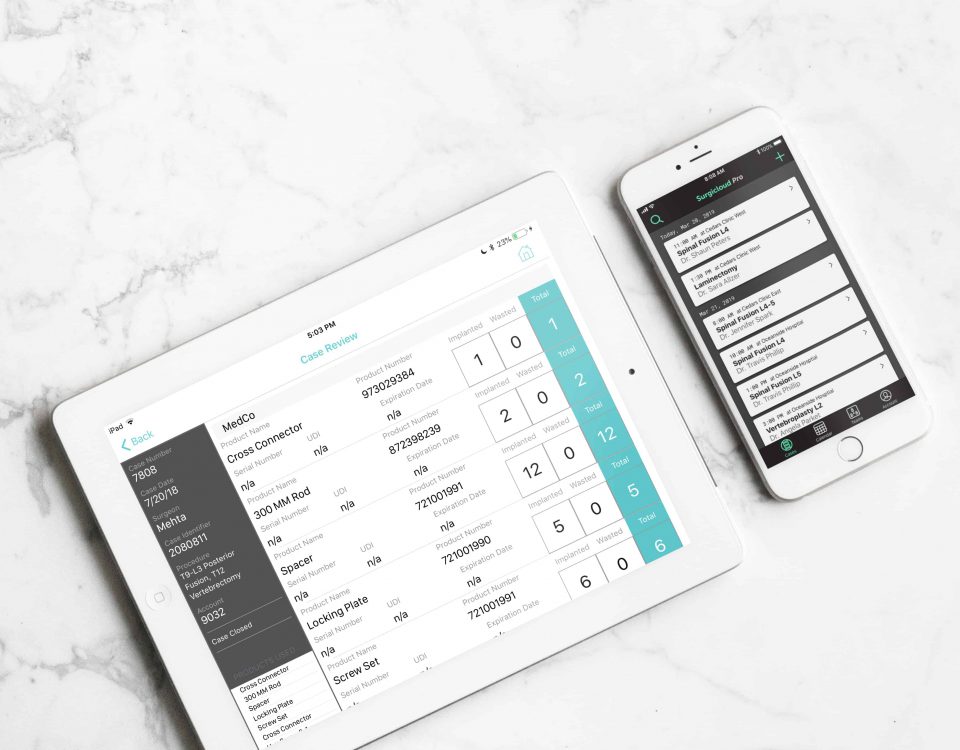
Surgicloud is a cloud-based inventory management, billing, and scheduling solution that optimizes and coordinates and device billing for hospitals’ surgical teams, medical device manufacturers, and distributors. Get in touch to request a demo today.